RSV Prevention Drug in Short Supply as Cases Rise
RSV Prevention Drug Rationed Amid Supply Shortage
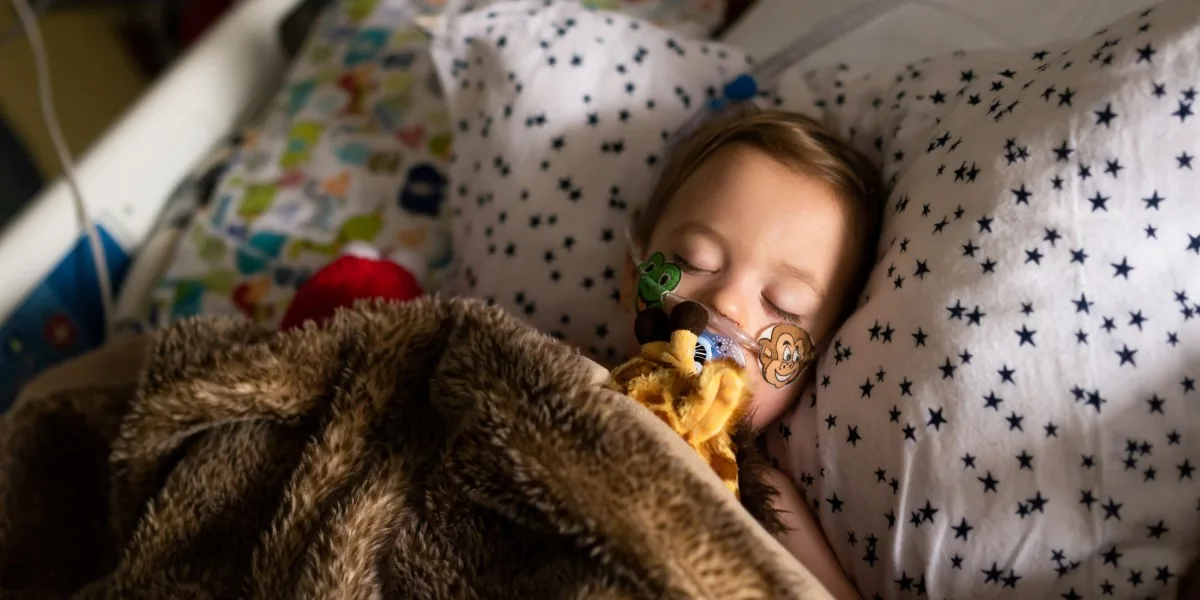
In the midst of a rise in cases of respiratory syncytial virus (RSV), a new preventative drug called nirsevimab is facing a significant shortage that has led to rationing. The Centers for Disease Control and Prevention (CDC) issued an advisory last week recommending pediatricians to reserve doses of nirsevimab for infants under six months of age and those with underlying conditions at highest risk for severe RSV. The scarcity of the drug has frustrated parents and pediatricians alike, as the manufacturer initially assured them that supply would not be an issue. A spokesperson for Sanofi, one of the companies involved in developing and marketing nirsevimab, mentioned that demand for the drug exceeded expectations based on historical pediatric immunization launches.
The Mismatch Between Supply and Demand
Foreseeing demand for preventive medicine like nirsevimab should have been relatively straightforward, considering the known number of births and timing. However, this situation is unique as nirsevimab is the first drug of its kind, making accurate forecasting challenging. Additionally, infants whose mothers have been recently vaccinated against RSV do not require the medication, further complicating demand calculations. Despite these uncertainties, the high demand for nirsevimab should not have come as a surprise. RSV, a respiratory virus causing cold-like symptoms, can be particularly dangerous for young children and older adults, with thousands of children being hospitalized and hundreds losing their lives to the illness every year. Last year’s surge in RSV cases prompted a state of emergency in some states.
The Benefits and Shortcomings of Nirsevimab
Nirsevimab, unlike a traditional vaccine, is a lab-made antibody administered via a shot and provides protection against RSV for approximately five months. Clinical trials have demonstrated that the drug can prevent a significant number of hospitalizations and intensive care admissions related to RSV. The drug’s efficacy and convenience—requiring only a single dose—make it a more favorable option compared to palivizumab, another monoclonal antibody available for RSV prevention. However, the limited supply of nirsevimab has left parents like Emi Ithen, who hoped to protect her six-month-old daughter from RSV, unsure of what alternative options exist. Manufacturing more doses will take time due to the complexity of producing monoclonal antibodies in bioreactors containing living cells.
Exploring Other Options for RSV Protection
While nirsevimab is currently the preferred choice for RSV prevention, there are other options available. Pregnant individuals can receive a new vaccine called Abrysvo between 32 and 36 weeks of pregnancy to pass on antibodies to their newborns. However, this method only benefits babies who have not yet been born. Palivizumab, another monoclonal antibody, has been in use for over two decades but is limited to babies born prematurely or those with specific risk factors for severe RSV. Unfortunately, it requires five separate shots over five months and is quite expensive. Parents like Ithen must wait for the supply of nirsevimab to catch up in order to protect their infants from the potential dangers of RSV.
Source: RSV is on the rise but preventative drugs are in short supply